Production of Materials > 2. Biomass Research >
Describe the reaction involved when a condensation polymer is formed
- Condensation polymers are one of two products made in condensation polymerisation reactions, the other product being a small molecule (often water).
- The formation of cellulose is an example of a condensation polymerisation reaction:
n(HO–C6H10O4–OH) → H–(O–C6H10O4)n–OH + (n – 1)H2O
- In the formation of cellulose, n glucose molecules combine to form the cellulose chain and (n – 1) molecules of water, which is in this case the small molecule product that is characteristic of such reactions.
- When two glucose monomers react, a hydroxyl group from each combine to condense a water molecule, leaving an oxygen atom linking the two monomers.
- This process repeats to form a chain.
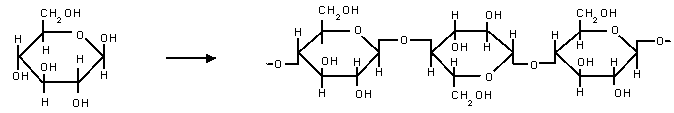
The condensation polymerisation reaction of glucose to cellulose
