[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Monitoring and Management > 3. Manufactured Products >
Describe the use of atomic absorption spectroscopy (AAS) in detecting concentrations of metal ions in solutions and assess its impact on scientific understanding of the effects of trace elements [/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]
- Spectroscopy: The study of the interaction of electromagnetic radiation with matter.
- When vaporised, different elements absorb light of specific frequencies.
- Atomic absorption spectroscopy (AAS): A technique used to identify the presence and concentration of substances by analysing the spectrum produced when a substance is vaporised and absorbs certain frequencies of light.
- AAS is used particularly for detecting the concentrations of metal ions in solutions.
- AAS is performed using an atomic absorption spectrometer.
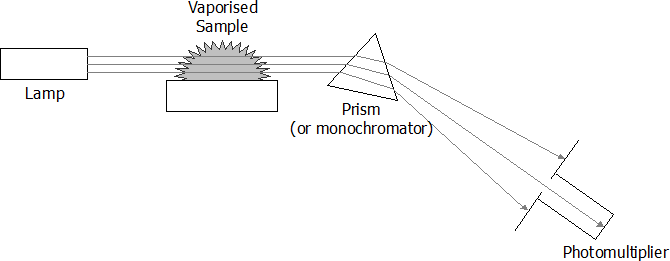
Schematic diagram of an atomic absorption spectrometer
- To determine the concentration of a certain metal ion in a sample, the following steps occur within an atomic absorption spectrometer:
- A hollow cathode lamp, with the cathode made of the metal to be tested for, emits light of a certain frequency.
- The light produced by the lamp is passed through the sample to be tested vaporised in a flame.
- The degree of light absorption is proportional to the concentration of the metal in the sample.
- The intensity of the light that passes through the flame is measured by a photomultiplier tube.
- By comparing the intensity with that produced from a control sample containing none of the metal ions being tested for, the degree of absorption, or absorbance, can be determined.
- The absorbance is then compared to that of a series of diluted standard solutions in order to determine the concentration.
- This involves the use of a calibration graph.
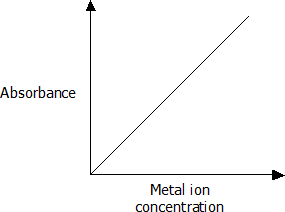
General layout of a calibration graph
-
- The standard solutions should produce a straight-line graph.
- The absorbance recorded for the sample being tested can be matched with a concentration using the graph.
- Trace element: Also known as a micronutrient, an element required in minute amounts for normal growth of organisms.
- Trace elements work in organisms by helping enzymes to function.
- The concentration of trace elements in animals and plants is normally in the range of 1 to 100 parts per million.
- Before the development of AAS in the 1950s, commonly used analytical methods were not sufficiently sensitive to detect the low concentrations of these elements, and their presence went unnoticed.
- When scientist began to use AAS on organisms and soils, the existence of these trace elements were first recognised.
- AAS has also been used to help demonstrate both the necessity and function of these elements.
- Thus, AAS has had a great impact on scientific understanding of the effects of trace elements.
- In the case of the ill health of an organism, AAS can be used to detect whether required trace elements are present in sufficient quantities in the organism and its environment.
- If a trace element deficiency is observed, then it can be rectified by providing the organism with that particular nutrient.
- This is especially useful in the field of agriculture, where specific practical applications of AAS have included:
- The discovery of a cobalt deficiency in seemingly good pastureland in coastal southwestern Australia where animal health could not be maintained.
- The discovery of a molybdenum deficiency in the soils of arid parts of Victoria where legume crops could not be supported.
[/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][x_video_embed no_container=”false” type=”16:9″][/x_video_embed][/cs_column][/cs_row][/cs_section][/cs_content]